Necrotizing enterocolitis (NEC), a potentially fatal gastrointestinal infection in premature babies, has been linked to the prominent cow’s milk baby formula Similac, according to more than four buyers complaints that led to filed lawsuits around the US vs Similac.
Currently, the law firm Miller & Zois is looking into possible claims against Abbott Laboratories for not warning parents about the dangers of NEC connected with their product. Families that are successful in their claim against NEC may be entitled to a sizable settlement.
What Is The Lawsuit About?
Infant nutrition products are widely marketed and used as an alternative to breast milk for babies who are unable or unwilling to receive it from their mothers.
Premature newborns who are fed cow milk-based products, including certain specialized milk, metabolic milk, and NEC and baby formula, have an increased chance of developing conditions like necrotizing enterocolitis. This is a life-threatening bowel infection that can pose serious risks to infants, particularly those who are premature. Medical science and FDA investigations have confirmed this.
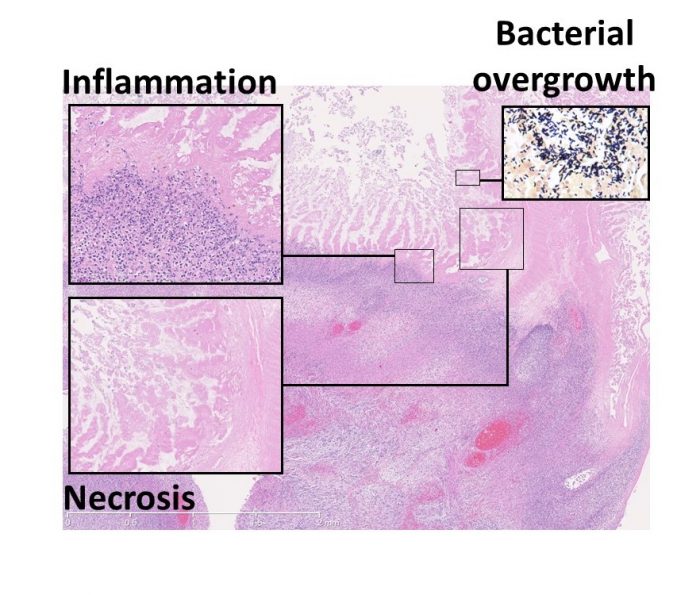
Infections due to contaminations in the gastrointestinal system of babies known as necrotizing enterocolitis (NEC) can rapidly degrade and kill the baby’s intestinal tissue. If you have necrotizing enterocolitis, it can begin in the large or small intestine and spread across the entire gut, starting in its inner layer and spreading outward.
The severity of NEC contamination cases varies. Some cases of necrotizing enterocolitis (NEC) are mild and only produce modest discomfort. Only a small fraction of newborns with NEC will experience symptoms that are so severe that they endanger their own lives. The bowels become inflamed and inflamed, resulting in extreme pain. Serious cases of NEC, if left untreated, can result in a rupture or hole forming in the intestinal wall.
If NEC results in a perforation of the intestine, the baby’s health is in jeopardy and may even be deadly. It is possible for bacteria from the intestines to enter the abdominal cavity through the perforation. This can lead to a severe bloodstream infection known as sepsis if it goes unchecked for too long.
NEC has been linked to powdered milk products for more than 30 years by scientific research. Cow milk-based formulas (including certain specialty formulas and metabolic formulas) have been shown to increase the risk of necrotizing enterocolitis (NEC) in premature infants compared to those receiving breast milk. The risk of NEC in premature babies is 10 times higher when they are fed a cow milk-based formula than when they are breastfed.
There are numerous public health groups, including the American Academy of Pediatrics, who strongly discourage the use of cow-milk powdered formula for premature babies and emphasize the responsibility of infant milk manufacturers to prioritize the health and safety of vulnerable infants.
Abbott Failures With Its Latest Formula Issue
Abbott Nutrition’s Sturgis facility or Michigan facility is the manufacturer of Similac powdered milk goods which include a wide range of formulas for full-term babies, preterm babies, and babies who need medical specialty infant milk. Abbott Nutrition products like this are accessible at retail stores, although some are exclusively available in hospitals.

It is Abbott Laboratories Inc. that manufactures and distributes the infant milk brand known as Similac, as well as other Abbott Nutrition products like EleCare powdered infant formulas. The aforementioned brand has been associated with an increased risk of necrotizing enterocolitis (NEC), one of the common infant illnesses. Abbott has been aware of this concern at their Sturgis facility for some time based on the environmental samples obtained.
While Abbott is well aware of the higher risk of NEC linked with certain powdered infant formulas, as well as infant milk shortages and recalls, parents and caregivers, including those participating in the Special Supplemental Nutrition Program for Women, Infants, and Children (WIC), have noted the absence of a warning label on these products. Each claim regarding powdered infant milk would focus primarily on Abbott’s refusal to issue a warning about the product.
Because Abbott is the manufacturer of the product, it is required by law to provide customers with information about any known side effects or hazards. NEC warnings, infant milk shortages, and Similac recalls have been omitted from the product labels of Abbott’s Similac and other liquid milk products, apparently to avoid adverse influence on Similac’s marketability. Enfamil vs. Similac is a fierce competition. Market share will be lost to the first powdered milk to produce an NEC warning.
Consequently, the Similac powdered milk cases, including Similac recall lawsuits, against Abbott are based on Abbott’s failure to notify consumers of the dangers of NEC and other issues related to their infant milk, including metabolic formulas and other Abbott Nutrition products.
What Will The Settlement Be For The Similac’s Lawsuits?
Legal proceedings in the Similac NEC case, milk shortage, and other related issues are still in their infancy. There has been no resolution to any of the NEC powdered formula cases, milk shortages, or trials. Neosure and Pro-Advance as well as other formulas from cows manufactured by Abbott for preemies are difficult to appraise because of this. Premature newborn NEC malpractice cases, special medical food complaints, and milk shortages have resulted in settlements of $3.5 million, according to lawyers. The immediate public health risk associated with these issues has prompted the FDA Commissioner Robert Califf to initiate immediate recall response activities and address concerns regarding bacterial infection and other health risks.
Formula Contamination
Buyers are advised not to buy recalled Similac Alimentum and EleCare powdered milk products after more than four consumer complaints have been filed. The 7 to 9-digit code and expiration date on the package’s bottom identify recalled products. Products are covered in the recalls if they include all three of the following:
- The code’s first two digits are 22 to 37
- Container code has K8, SH, or Z2
- The expiration date of 4-1-2022 (APR 2022) or later.
Recalled powdered baby formula products — including Similac Alimentum and EleCare — may contain Cronobacter infection, a bacterium that can cause serious food poisoning, especially in young children. Although they are uncommon, Cronobacter infections are particularly dangerous for newborn babies.
A serious infection (sepsis) or meningitis can be caused by salmonella bacteria and Cronobacter bacteria. Neonatal meningitis, necrotizing enterocolitis, and bacteremia are all caused by the salmonella bacteria and Cronobacter sakazakii. Ventriculitis and brain abscesses can occur in patients who survive the first infection. Contaminated powdered infant formula products that cause Cronobacter infection have been shown to contain C. sakazakii.

- Infants with Cronobacter from similac formula contamination typically present with a fever, excessive crying, poor feeding, or low energy at the beginning of their infant illness related problem. Some infants may also experience due to Similac formula contamination are seizures, yellow skin, etc. Babies who exhibit these signs should visit a doctor as soon as feasible.
- Cronobacter bacteria from Similac formula contamination can enter the bloodstream or cause swelling in the lining around the brain and spine in babies, particularly those under two months old (meningitis).
- Due to Similac formula contamination, infants with meningitis may experience yellow skin and severe, lifelong brain issues. When a baby has Cronobacter meningitis, up to 4 out of 10 of them could pass away.
- Your child’s healthcare professional should be notified promptly if any of these indications are present in your child after consuming the newest Similac Formula. Cronobacter sakazakii should be reported to the CDC by healthcare practitioners and health departments.
Bookmark Family Hype to know updates on certain powdered infant formula lawsuits.
Similac Formula
FAQS
What Problems Was Similac Causing?
Similac’s new formula was causing problems because it was contaminated with Cronobacter sakazakii bacteria, leading to a powdered infant formula investigation, recalls of the formula, and baby formula lawsuits.
Is It Safe To Use Similac PM 60 40 Or Any Similac Formulation?
What Powdered Infant Formula Has Been Recently Recalled?
Is Similac PM 60 40 Still On Voluntary Recall 2022?
What Is The Safest Baby Formula 2024?
What Brands Of Similac Are Recalled By Disease Control?
How Do I Know If My Baby Is Affected By The Contaminated With Cronobacter Sakazakii?
Will Similac Replace Recalled Powdered Infant Formula?
How Do I Know If My Similac Was Part Of The Voluntary Recall?
How Do You Know If A Formula Is Contaminated?
What Powdered Infant Formula Is Being Recalled 2023 By Disease Control?
Federal health officials recalled formula due to contamination with Cronobacter Sakazakii infection, including Similac new formula, and Abbott formula plant.
Is Similac Formula Safe Now?
Yes, Similac Formula is safe now; however, parents should still be cautious and follow guidelines provided by medical providers and FDA investigators, especially after recalls by Abbott Nutrition and Mead Johnson due to dangerous bacteria in some specialty formulas, and they should avoid buying formula online or feeding their baby’s formula contaminated with herbal teas, particularly after the incident involving a Missouri infant.
Is Similac Better Than Enfamil?
It’s important to see a medical provider regarding formula feeding, as both Similac and Enfamil have faced issues like contamination, but homemade infant formula or dry foods can be alternatives, considering Abbott and Mead Johnson, the manufacturers, and potential recalls.
What Are The Disadvantages Of Similac Milk?
Disadvantages of Similac milk may include the risk of contamination with the new formula, leading to potential health issues for infants who consume the product, prompting caution when buying formula online, and recommending seeing state and local health department partners or the local WIC clinic for guidance, as regular or similar formula options might be safer alternatives as completed laboratory testing has found naturally.
Can I Switch My Baby From Similac To Enfamil?
Yes, you can switch your baby from Similac to Enfamil, but be aware that Abbott Nutrition recalled some Similac new formula due to contamination in Sturgis, Michigan, where four infants drank the infant formula, leading to adverse events; consider completing laboratory testing before purchasing formula online, and if needed, consult a healthcare provider for specialty formula recommendations.
Last Updated on April 12, 2023 by Raymond Sy Tamco
DISCLAIMER (IMPORTANT): This information (including all text, images, audio, or other formats on FamilyHype.com) is not intended to be a substitute for informed professional advice, diagnosis, endorsement or treatment. You should not take any action or avoid taking action without consulting a qualified professional. Always seek the advice of your physician or other qualified health provider with any questions about medical conditions. Do not disregard professional medical advice or delay seeking advice or treatment because of something you have read here a FamilyHype.com.